Pharmaceutical Turnkey Solution
| Place of Origin:Zhejiang China (Mainland) | Brand Name:HM PHARMACHINE | Model Number:Turnkey project | Usage:Liquid |
| Pharmaceutical:turnkey project | Application:Pharma | Standards:GMP/ cGMP/ FDA | Certification:CE/ ISO 9001 |

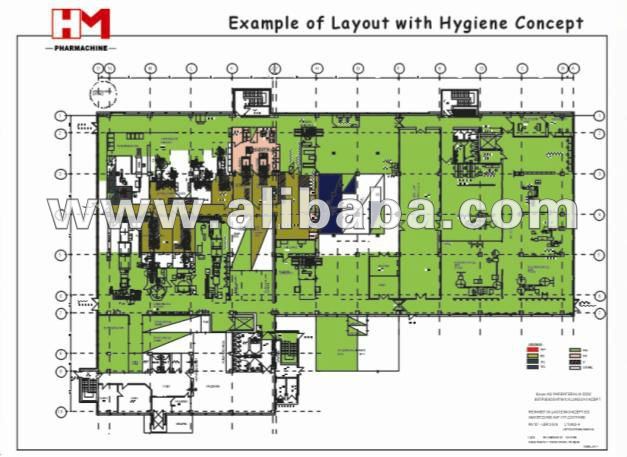
HM Pharmaceutical Project Engineering Design
Joint advantage:Europe and USA engineering companies provide project consultation and Cgmp concept design; China design companies give basic design and construction detailed design.
Project Engineering Design Clients Wanted
Complying with EU Cgmp/ FDA/WHO GMP/ MHRA/ TGA regulations, can get approval and enter into international market.
Suitable for developing countries, reasonable investment, easy implement and operational design
High-effective economical project engineering service.
Good investment return, long term profitable project.
HM Pharmaceutical Project Engineering Design
WHO, USAID experienced GMP auditing expert, provide project design full complying with EU cGMP/ FDA/ WHO GMP/ MHRA/ TGA regulations. Frequently GMP auditing and assessing of USA, Germany, Italy, Japan, Bulgaria, Egypt, South Africa, Syria, Indonesia, Korea, China etc worldwide pharmaceutical companies. Deep and precise understanding of worldwide GMP standard and validation requirement.
Europe cGMP concept design, combine with China basic design and detailed design. Provides operational design suitable for developing countries, most of equipments, device, and facilities are high end China made, economical project investment, competitive and operational, project can be implement easily and quickly.
High-effective economical project engineering service, shortens the design period and saves the design cost. 2-3 months for cGMP concept design, 6 months to complete all engineering design.
Over 30 years experience of Europe international pharmaceutical projects, Provides dedicated engineering design, not only complying with EU/ USA/ cGMP regulation, environment and safety guideline, but also considering the project benefits, from concept design to save project investment and running cost, acaonsidering 6-10 years long term expansion plan, give flexible space for new tecgnology and product development.
| Packaging Detail:Suitable for transportation packaging. |
| Delivery Detail:45 Days |














